
"Mad Cow Disease"

"Mad Cow Disease"
Bovine spongiform encephalopathy (BSE) is a fatal brain disease of cattle. The disease is believed to be caused by a "self-replicating" protein (a prion) rather than a bacterium or virus. Meat and milk have not been shown to carry the infective agent and measures have been taken to exclude those parts shown to carry the infective agent (primarily brain and nervous tissue) from the food supply. Mad cow disease leaves spongy holes in the brain and is thought to be caused by a protein-gone-bad known as a prion. The latest test revealed that the prion in mad cow disease matches the one in humans with the new strain of CJD (Creutzfeldt-Jakob disease).
Prions - The Disease Causing Agent
The disease appears to be caused by an unconventional infectious agent, originally described as a "slow virus," a "self-replicating protein" and more recently as a "prion", an aberrant conformation of normal protein (PrP). This agent is extremely resistant to heat and to normal sterilization processes. It does not evoke a detectable immune response or inflammatory reaction in host animals.
The prion protein, PrP, is a normal cell protein present on nerve cell membranes. The gene for PrP is present in most mammals. However, its normal function is unclear; mice without the protein appear to be fine.
The sequence of amino acids in PrP and PrPsc is identical. This means PrPsc is changed after synthesis from the gene. The change is in conformation or shape. PrP changes conformation, from predominantly helix to predominantly pleated sheet, creating PrPsc.
The altered PrPsc protein is catalytic or "self-propagating". One PrPsc causes other PrP molecules to change into PrPsc. The PrPsc protein is very resistant to endogenous protease that would normally destroy the protein. Because the PrPsc can't be broken down, it builds up, aggregates, then precipitates forming plaques and causing spongiform damage.
For the same reason that cells can not destroy PrPsc, prions are very heat resistant. While some loss of infectivity occurs at temperatures above 100°C, 30 to 60 minutes at more than 130°C is needed for inactivation. Prions remain infective after: sterilizing levels of radiation, formalin, extremes of pH, non-polar organic solvents, burying for years, passing through 0.1 µm filters (2.2 µm filters remove bacteria). Prions infectivity is destroyed by: 1M NaOH @ 55°C , chlorine bleach @ 20,000 ppm (household bleach is 50,000 ppm) hypochlorite.
What causes the original change in conformation of normal PrP to disease causing PrPsc? While the spontaneous conversion of PrP to PrPsc is unfavorable (corresponding to a low incidence of sporadic CJD), mutations in the PrP gene can make the spontaneous conversion more likely. There are at least 20 known mutations in the PrP gene sequence resulting in spontaneous PrPsc formation. Each mutation results in a slightly different conformation, and slightly different pathology. This results in different prion strains. Regardless of the mutation the spontaneous PrPsc can also change non-mutated PrP into PrPsc.
Creutzfeldt-Jakob disease (CJD) is a related prion disease in humans. Almost all cases of CJD are spontaneous, inherited or iatrogenic. A small number of variant CJD cases have been linked to BSE exposure. Stanley B. Prusiner, University of California was recently awarded a Nobel Prize for his work on prions.
BSE, a transmissible spongiform encephalopathy (TSE) of cattle, was first observed in Great Britain in April 1985, and was specifically diagnosed in 1986. By June 1990, there were some 14,000 confirmed cases out of an estimated population of 10 million cattle in Great Britain.

The gross findings in kuru are minimal, and consist of congestion of blood vessels, which can be seen on this slide, and in long standing cases, cortical atrophy (not prominent in this case).

Another characteristic finding of kuru is shown on this slide: brush-like plaques, which are also called spikeballs and found in the cerebellum.
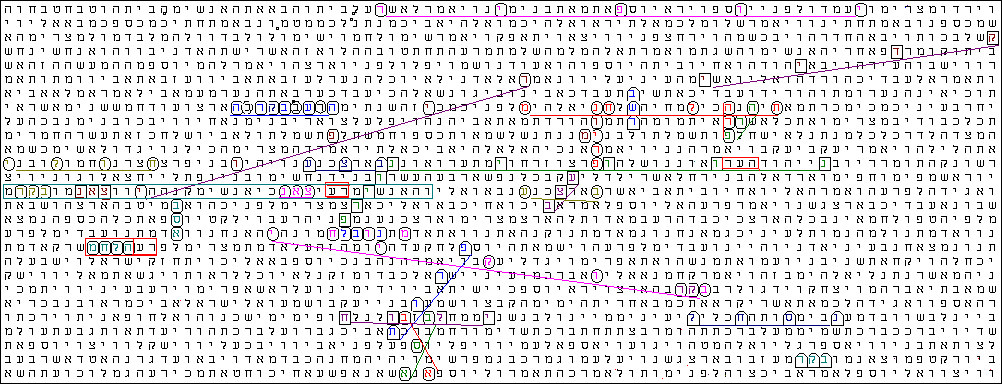
Bible Code Matrix This Code array shows the Hebrew of the Torah text from Genesis 43:15 to Genesis 50:17
Central Term : PRION ( ELS -447 )

|
CODE ANALYSIS The words encoded many times at low or minimal intervals ; and have the exact same interval.
Some of the key words share a common letter and share some common space.***an important point is that in close proximity to the word Prion we found the word Protein & Brain
Words with a Common Letters
- Protein shared with the word Brain, the letter Vav & Chet
- the words Transmissible, Disease & Cattle have in common the letters: Hey, Bet, Resh, Ayin, Hey
- The word Prion shared with the word Brain the letter VAV
- Cow & Cow have in common the letter Pe Ef
- Nerve & Nerve share the letter Tzadik
- BSE & BSE have in common the letter Samech
- the word Cows crossed the word BSE & Morbos
- The word Atrophy Crossed the word Prion & shared with it the letter Nun
 |

|
the evil "Disease" in or of the Cattle -Transmissible Prion Protein
|

|
Prion Disease Flesh Cow Cow Brain Nerve Nerve Prion shared with the word Brain the letter VAV Cow & Cow they have in common the letter PEY Nerve & Nerve shared with the letter TZADIK Disease crossed the central term Prion & Cow Brain crossed the central term Prion |
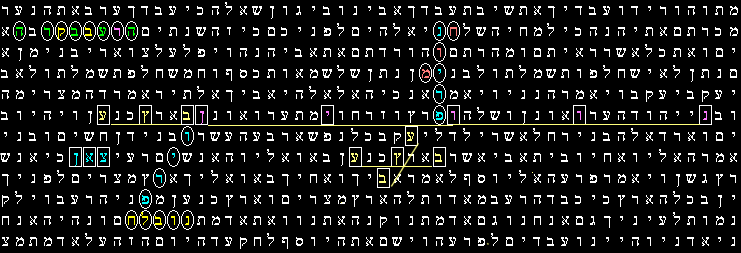
The Evil "Disease" in the Cattle -Transmissible Prion Protein Brain Nerve Nerve Atrophy "Degeneration"
|